Once-daily tiotropium improved lung function when added to ICS maintenance therapy in adolescents with moderate symptomatic asthma
This 48-week study was conducted at 65 sites in 12 countries and included 398 patients aged 12 to 17 years. They were randomized to receive 5 μg (2 puffs of 2.5 μg) or 2.5 μg (2 puffs of 1.25 μg) of once-daily tiotropium or placebo (2 puffs) administered through the Respimat device every evening, each as add-on treatment to inhaled corticosteroid (ICS) background therapy, with or without a leukotriene receptor antagonist. Of note, long-acting beta-2-agonists were not permitted during the study.
The primary end point was improvement in peak FEV1 within 3 hours after tiotropium dosing at 24 weeks, and it was achieved during the study. Trends for improvement in asthma control and health-related quality of life over the 48-week treatment period were observed.
Once-daily tiotropium improved lung function and was well tolerated when added to ICS maintenance therapy in adolescents with moderate symptomatic asthma. The effect was larger with the higher tiotropium dose (5 μg).
Here is what wheezing sounds like (click to play the embedded video):
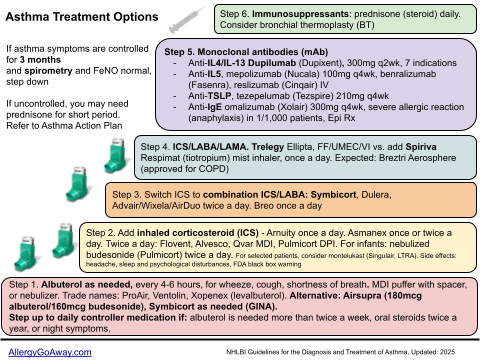
Asthma Treatment Options in 7 Steps (click to enlarge the image).
References:
Tiotropium add-on therapy in adolescents with moderate asthma: A 1-year randomized controlled trial. Eckard Hamelmann et al. JACI, August 2016, Volume 138, Issue 2, Pages 441–450.e8 (free full text).
http://www.jacionline.org/article/S0091-6749(16)00186-X/fulltext
The World Allergy Organization (WAO) Small Airways Working Group publishes a monthly "What's New?" summary and I have served as its editor since 2011. The summary features the top 3 asthma/small airways articles each month. The article above is a part of the project. The archive is here: http://www.worldallergy.org/small_airways_group/reviews/archive.php
Image source: Image source: FDA and Wikipedia, public domain.
The primary end point was improvement in peak FEV1 within 3 hours after tiotropium dosing at 24 weeks, and it was achieved during the study. Trends for improvement in asthma control and health-related quality of life over the 48-week treatment period were observed.
Once-daily tiotropium improved lung function and was well tolerated when added to ICS maintenance therapy in adolescents with moderate symptomatic asthma. The effect was larger with the higher tiotropium dose (5 μg).
Here is what wheezing sounds like (click to play the embedded video):
Asthma Treatment Options in 7 Steps (click to enlarge the image).
References:
Tiotropium add-on therapy in adolescents with moderate asthma: A 1-year randomized controlled trial. Eckard Hamelmann et al. JACI, August 2016, Volume 138, Issue 2, Pages 441–450.e8 (free full text).
http://www.jacionline.org/article/S0091-6749(16)00186-X/fulltext
The World Allergy Organization (WAO) Small Airways Working Group publishes a monthly "What's New?" summary and I have served as its editor since 2011. The summary features the top 3 asthma/small airways articles each month. The article above is a part of the project. The archive is here: http://www.worldallergy.org/small_airways_group/reviews/archive.php
Image source: Image source: FDA and Wikipedia, public domain.