The US Food and Drug Administration (FDA) approved the first topical ocular formulation of the antihistamine cetirizine for the treatment of ocular itching associated with allergic conjunctivitis.
The efficacy of cetirizine ophthalmic solution 0.24% (Zerviate) was shown in 3 clinical trials.
Patients had less ocular itching within 15 minutes and the effect persisted for 8 hours after treatment.
In PR-focused wording, the company quotes cetirizine "world-wide exposure representing more than 300 million patient-years".
The recommended dose of Zerviate is one drop in each affected eye twice daily, 8 hours apart.
The most commonly reported adverse reactions were ocular hyperemia (redness), instillation site pain, and reduction in visual acuity, which occurred in roughly 1% to 7% of patients.
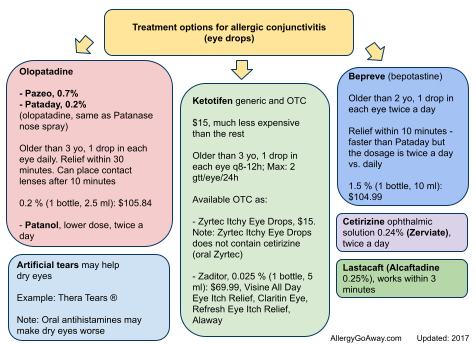
Treatment options for allergic conjunctivitis (eye drops) (click here for larger image).
References:
FDA Clears Cetirizine Eye Drops for Allergic Conjunctivitis http://buff.ly/2swY4Sp
The efficacy of cetirizine ophthalmic solution 0.24% (Zerviate) was shown in 3 clinical trials.
Patients had less ocular itching within 15 minutes and the effect persisted for 8 hours after treatment.
In PR-focused wording, the company quotes cetirizine "world-wide exposure representing more than 300 million patient-years".
The recommended dose of Zerviate is one drop in each affected eye twice daily, 8 hours apart.
The most commonly reported adverse reactions were ocular hyperemia (redness), instillation site pain, and reduction in visual acuity, which occurred in roughly 1% to 7% of patients.
Treatment options for allergic conjunctivitis (eye drops) (click here for larger image).
References:
FDA Clears Cetirizine Eye Drops for Allergic Conjunctivitis http://buff.ly/2swY4Sp