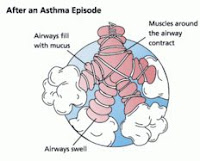
 Bronchial thermoplasty (BT) is a new bronchoscopic procedure which involves the application of radiofrequency to reduce the mass of airway smooth muscle and attenuate bronchoconstriction.
Bronchial thermoplasty (BT) is a new bronchoscopic procedure which involves the application of radiofrequency to reduce the mass of airway smooth muscle and attenuate bronchoconstriction.In April 2010, the FDA Approved Alair Bronchial Thermoplasty System for Adults with Severe and Persistent Asthma.
In a study of 16 patients, BT resulted in decreased airway hyperresponsiveness that persisted for at least 2 yr. In a study of 122 patients, BT was safe and resulted in a significant improvement in asthma outcomes in patients with severe refractory asthma.
A video animation of bronchial thermoplasty using the Alair® System is available from the manufacturer website. The system has an expandable wire basket at the tip that comes in contact with and fit snugly against the airway wall. The expanded basket delivers controlled radio frequency energy for about 10 seconds to heat the airway smooth muscle.
BT is an outpatient bronchoscopic procedure with conscious sedation. The full treatment requires 3 procedures on 3 separates days. Each procedure lasts about 30-60 minutes.
The Alair® Bronchial Thermoplasty (BT) System is the first device-based asthma treatment approved by the FDA:
Bronchial Thermoplasty. Dr. Kyle Hogarth, MD, assistant professor of medicine at the University of Chicago Medical Center, talks about bronchial thermoplasty, a new treatment for patients with advanced, hard-to-control asthma. Hogarth describes the rationale behind the procedure, what the patient experiences (3-session procedure, conscious sedation similar to the one used during colonoscopy), and the expected outcome (discharge after 1-2 hours, worse symptoms for 2 days, improved symptoms after 2 weeks). UChicagoMedCenter | September 14, 2010.
Randomized controlled clinical trials of BT in severe asthma have not been able to show a reduction in airway hyperresponsiveness or change in FEV1 but have suggested an improvement in quality of life, as well as a reduction in the rate of severe exacerbations, emergency department visits, and days lost from school or work. Strict inclusion and exclusion criteria of these trials resulted in the elimination of patients with severe asthma who experienced more than three exacerbations per year. Therefore, the generalizability of this treatment to the broader severe asthma population still needs to be determined (Am. J. Respir. Crit. Care Med. April 1, 2012).
References:
Bronchial thermoplasty for asthma: A critical review of a new therapy. Wechsler, Michael. Allergy and Asthma Proceedings, Volume 29, Number 4, 7/8 2008 , pp. 365-370(6).
RCTs of Bronchial Thermoplasty for Severe Asthma haven't shown reduction in airway hyperresponsiveness or FEV1 change http://goo.gl/mX5Bv, Am. J. Respir. Crit. Care Med. April 1, 2012 vol. 185 no. 7 709-714.
Bronchial Thermoplasty for Asthma. Gerard Cox, John D. Miller, Annette McWilliams, J. Mark FitzGerald and Stephen Lam. American Journal of Respiratory and Critical Care Medicine Vol 173. pp. 965-969, (2006).
Asthma Control during the Year after Bronchial Thermoplasty. Gerard Cox, M.B., Neil C. Thomson, M.D., Adalberto S. Rubin, M.D., Robert M. Niven, M.D., Paul A. Corris, M.D., Hans Christian Siersted, M.D., Ronald Olivenstein, M.D., Ian D. Pavord, M.D., David McCormack, M.D., Rekha Chaudhuri, M.D., John D. Miller, M.D., Michel Laviolette, M.D., for the AIR Trial Study Group. NEJM, Volume 356:1327-1337, March 29, 2007, Number 13.
PowerPoint presentation:
Airway remodeling in asthma. Pierre Ernst. McGill University.
In a study of 16 patients, BT resulted in decreased airway hyperresponsiveness that persisted for at least 2 yr. In a study of 122 patients, BT was safe and resulted in a significant improvement in asthma outcomes in patients with severe refractory asthma.
A video animation of bronchial thermoplasty using the Alair® System is available from the manufacturer website. The system has an expandable wire basket at the tip that comes in contact with and fit snugly against the airway wall. The expanded basket delivers controlled radio frequency energy for about 10 seconds to heat the airway smooth muscle.
BT is an outpatient bronchoscopic procedure with conscious sedation. The full treatment requires 3 procedures on 3 separates days. Each procedure lasts about 30-60 minutes.
The Alair® Bronchial Thermoplasty (BT) System is the first device-based asthma treatment approved by the FDA:
Bronchial Thermoplasty. Dr. Kyle Hogarth, MD, assistant professor of medicine at the University of Chicago Medical Center, talks about bronchial thermoplasty, a new treatment for patients with advanced, hard-to-control asthma. Hogarth describes the rationale behind the procedure, what the patient experiences (3-session procedure, conscious sedation similar to the one used during colonoscopy), and the expected outcome (discharge after 1-2 hours, worse symptoms for 2 days, improved symptoms after 2 weeks). UChicagoMedCenter | September 14, 2010.
Randomized controlled clinical trials of BT in severe asthma have not been able to show a reduction in airway hyperresponsiveness or change in FEV1 but have suggested an improvement in quality of life, as well as a reduction in the rate of severe exacerbations, emergency department visits, and days lost from school or work. Strict inclusion and exclusion criteria of these trials resulted in the elimination of patients with severe asthma who experienced more than three exacerbations per year. Therefore, the generalizability of this treatment to the broader severe asthma population still needs to be determined (Am. J. Respir. Crit. Care Med. April 1, 2012).
References:
Bronchial thermoplasty for asthma: A critical review of a new therapy. Wechsler, Michael. Allergy and Asthma Proceedings, Volume 29, Number 4, 7/8 2008 , pp. 365-370(6).
RCTs of Bronchial Thermoplasty for Severe Asthma haven't shown reduction in airway hyperresponsiveness or FEV1 change http://goo.gl/mX5Bv, Am. J. Respir. Crit. Care Med. April 1, 2012 vol. 185 no. 7 709-714.
Bronchial Thermoplasty for Asthma. Gerard Cox, John D. Miller, Annette McWilliams, J. Mark FitzGerald and Stephen Lam. American Journal of Respiratory and Critical Care Medicine Vol 173. pp. 965-969, (2006).
Asthma Control during the Year after Bronchial Thermoplasty. Gerard Cox, M.B., Neil C. Thomson, M.D., Adalberto S. Rubin, M.D., Robert M. Niven, M.D., Paul A. Corris, M.D., Hans Christian Siersted, M.D., Ronald Olivenstein, M.D., Ian D. Pavord, M.D., David McCormack, M.D., Rekha Chaudhuri, M.D., John D. Miller, M.D., Michel Laviolette, M.D., for the AIR Trial Study Group. NEJM, Volume 356:1327-1337, March 29, 2007, Number 13.
PowerPoint presentation:
Airway remodeling in asthma. Pierre Ernst. McGill University.
FDA Approves Alair Bronchial Thermoplasty System for Adults with Severe and Persistent Asthma, 2010.
YouTube - New Asthma Treatment Available at Henry Ford Hospital http://goo.gl/X7I2

Severe asthma - differential diagnosis and management (click to enlarge the image).
Related reading:
Study: Bronchial thermoplasty decreases asthma attacks by 32% and ER visits by 84% in patients with severe asthma. http://is.gd/B76h
Image source: Wikipedia, public domain.
YouTube - New Asthma Treatment Available at Henry Ford Hospital http://goo.gl/X7I2
Severe asthma - differential diagnosis and management (click to enlarge the image).
Related reading:
Study: Bronchial thermoplasty decreases asthma attacks by 32% and ER visits by 84% in patients with severe asthma. http://is.gd/B76h
Image source: Wikipedia, public domain.